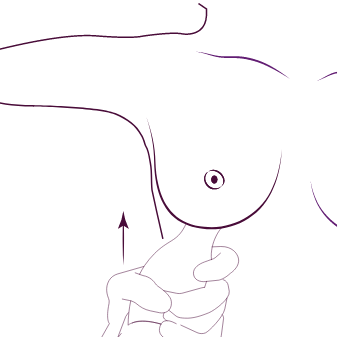
Bevara deras bröstanatomi
Preservé™ introducerar en innovativ teknik för skonsam bröstkirurgi som bevarar patientens ursprungliga bröstvävnad och känsel över tid*.1

Preservé™ introducerar en innovativ teknik för skonsam bröstkirurgi som bevarar patientens ursprungliga bröstvävnad och känsel över tid*.1

Genom att kombinera SmoothSilk® Ergonomix2®-implantatet med Preservé™:s egenutvecklade verktyg för att komplettera Preservé™-systemet får patienterna mindre ärr, färre komplikationer relaterade till implantatet utan att den ursprungliga bröstvävnaden störs, vilket ger en snabb och säker återhämtning.2

Vår mindre invasiva* preserveringsteknik minimerar skadorna på kvinnans naturliga bröststrukturer genom att förlänga de ursprungliga vävnaderna så att de kan placeras runt implantatet. Detta tillvägagångssätt förbättrar stabiliteten, vilket framgår av en klinisk studie som rapporterade en 0%-ig frekvens av inferior malposition efter tre år.1

Genom att kombinera Motiva SmoothSilk®-ytan med Preservé™ är denna metod utformad för att minimera vävnadspåverkan och främja ett lågt inflammatoriskt svar. Detta förfarande ger sinnesfrid, uppbackat av kliniska bevis som inte visar några komplikationer såsom proliferativa sjukdomar (som BIA-ALCL). Preservé™:s garantipaket ger dessutom patienten stöd i den sällsynta händelsen av en enhetsrelaterad komplikation eller ett oönskat resultat.4
*Jämfört med traditionell bröstförstoring.




1. Tunnling med Motiva®
kanalseparator kanalseparator
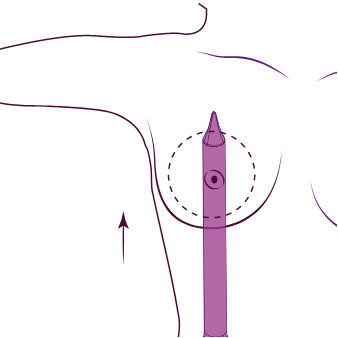
2. Kontrollerad vävnadsförlängning med
Motiva® uppblåsbar
ballong
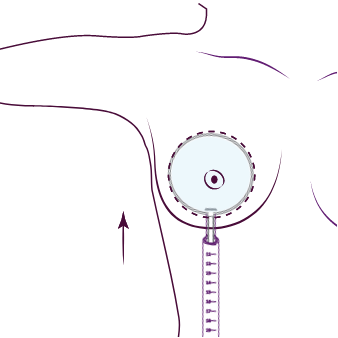
3. Utplacering av SmoothSilk® Ergonomix2® med Motiva med Motiva® införingshylsa